Abstract
Introduction: Hodgkin lymphoma (HL) is a rare disease that commonly occurs in AYAs, defined in the United States as patients 15 to 39 years of age. Brentuximab vedotin (Adcetris®; A) is an anti-CD30 antibody-drug conjugate approved for adult patients with previously untreated stage III or IV classical HL (cHL) in combination with doxorubicin, vinblastine and dacarbazine (AVD) chemotherapy based on results from the phase 3 ECHELON-1 trial which demonstrated a significantly improved modified progression-free survival (mPFS) compared with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (HR, 0.77; 95% CI, 0.60-0.98; P=0.04) (Connors JM, et al. N Engl J Med. 2018;378:331-344). Here we describe key efficacy and safety results for 18-to-39-year-old AYA patients enrolled in ECHELON-1.
Methods: ECHELON-1 is a global, open-label, multicenter, randomized trial of patients with previously untreated stage III or IV cHL. Patients ≥18 years of age enrolled from both academic and community sites were randomized to receive A+AVD (n=664) or ABVD (n=670). The primary endpoint was mPFS, defined as progression, death, or receipt of additional anticancer therapy for patients who were not in complete response after completion of frontline therapy, as adjudicated by an independent review facility (IRF). Safety and tolerability was also assessed. To account for regional differences in the age ranges that define AYA patients, this exploratory subgroup analysis compares efficacy and key safety outcomes for AYA patients <30 and ≤39 years of age.
Results: The AYA population consisted of 771 patients comprising 57.8% of the total trial population; the cohort comprised 303 patients aged 30 to 39 years, and 468 patients aged <30 years including 137 patients aged 18 to 21 years, 171 patients aged 22 to 25 years, and 160 patients aged 26 to 29 years. In patients aged <30 years, 244 received A+AVD and 224 received ABVD; in those ≤39 years of age, 396 received A+AVD and 375 received ABVD. Baseline demographics and disease characteristics were similar across age groups and treatments. The proportion of AYA patients treated in the Americas (40%), Europe (50%) and Asia (9%) reflected those of the overall trial population.
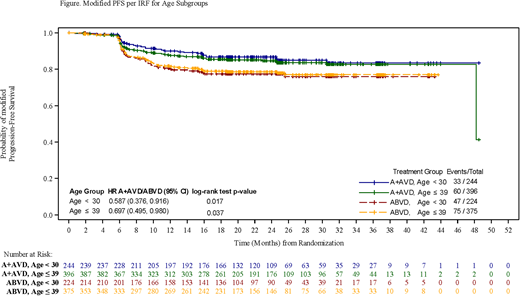
Consistent with the overall trial population, median follow-up time was approximately 24 months for both age groups. AYA patients receiving A+AVD had an improved overall mPFS compared with patients receiving ABVD (≤39 years of age, HR 0.697 [95% CI, 0.495-0.980]; <30 years of age, HR 0.587 [95%CI, 0.376-0.916]) (Figure). The 2-year mPFS, also per IRF, for patients ≤39 years who received A+AVD was 84.6% vs 78.6% for those who received ABVD; patients aged <30 years receiving A+AVD also had improved 2-year mPFS compared with patients receiving ABVD (86.7% vs 74.4% respectively). Conventional PFS, as measured by investigators, was consistent with mPFS results for each age group: the HR for PFS in patients receiving A+AVD vs ABVD was 0.652 (95% CI, 0.449-0.945; P=0.023) in patients ≤39 years of age and 0.595 (95% CI, 0.367-0.965; P=0.033) in patients aged <30 years.
The rate of treatment-emergent adverse events (TEAEs) in patients receiving A+AVD or ABVD was similar across age groups and reflected that of the overall trial population. TEAEs of interest include peripheral neuropathy (PN), febrile neutropenia (FN), and pulmonary-related toxicity (PRT). For patients receiving A+AVD or ABVD, any-grade PN occurred 64% and 40% of AYA patients; the majority (69%) of patients with PN had improvement/resolution of PN. The rate of FN in patients on A+AVD or ABVD arms was 16% and 5%, respectively; the incidence of FN was lower in patients who received G-CSF primary prophylaxis (9% and 5%, respectively). The incidence of PRT was low on both A+AVD (2%) and ABVD arms (4%).
Conclusions: This exploratory analysis of ECHELON-1 demonstrated that AYAs receiving A+AVD had significantly improved mPFS with a manageable tolerability profile compared with AYA patients receiving ABVD. A+AVD can be considered a treatment option for AYAs with advanced-stage cHL. Additional data presented will focus on comparing results between AYA age ranges.
Crosswell:Seattle Genetics: Other: stock ownership in Seattle Genetics. LaCasce:Research to Practice: Speakers Bureau; Humanigen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Bristol-Myers Squibb: Other: Data safety and monitoring board. Bartlett:Affimed: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Bristol-Meyers Squibb: Research Funding; Merck & Co: Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Novartis: Research Funding; Immune Design: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Research Funding; Novartis: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Millennium: Research Funding; ImaginAB: Research Funding; Forty Seven: Research Funding. Straus:Medical Crossfire: Speakers Bureau; DAVA Oncology: Consultancy, Honoraria; InPractice Elselvier: Consultancy; JUNO: Consultancy; Bayer: Consultancy; Roch China: Speakers Bureau; Seattle Genetics: Consultancy; Memorial Sloan Kettering Cancer Center: Employment; Onco Tracker: Consultancy; Millenium (Takeda): Consultancy, Research Funding. Fenton:Seattle Genetics, Inc.: Employment, Equity Ownership. Engley:Seattle Genetics: Employment. Jolin:Takeda Pharmaceuticals International Co.: Employment. Liu:Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Zinzani:TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Collins:Gilead: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Amgen: Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Research Funding; Celleron: Consultancy, Honoraria; MSD: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Celgene Corporation: Research Funding; Pfizer: Consultancy, Honoraria. Grigg:Roche: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal